Welcome
Discover the new service platform for Pierre Fabre Group brands, dedicated to professionals in the medical sector.
Headlines
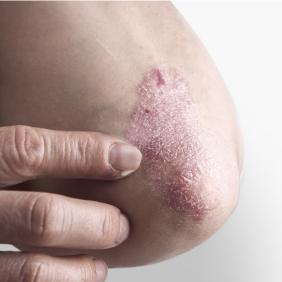
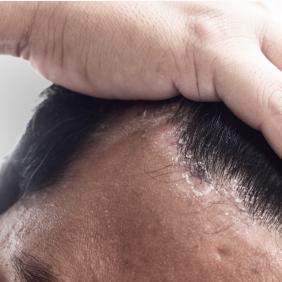
Pierre Fabre, the dermatological expertise
Our laboratory studies numerous dermatological conditions for which we offer solutions. Find out more about our expertise and our solutions for each of them.
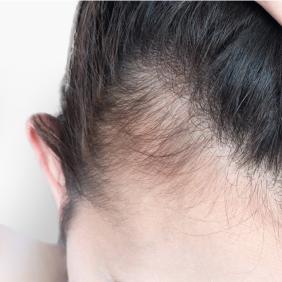
... in every field
Dermatology cuts across many areas of medicine. Find out how our expertise and care can help you in your speciality.
Our brands

Since 1930, Ducray Dermatological Laboratories have been innovating in the fields of skin and hair. This dual expertise supports doctors and their patients on a daily basis.

Eau Thermale Avène offers skincare products for all skin types, even the most sensitive. At the heart of its products is Avène Thermal Spring Water, a unique active ingredient that helps restore the skin barrier.

A-DERMA, our plant-based laboratory, uses the rebalancing and soothing properties of dermatological oatmeal to care for very dry skin with atopic tendencies.

Discover the Dexeryl® Range; Essential Expert Care for Dry Skin. The Dexeryl® Range gently cleanses, moisturises, nourishes, soothes, relieves and repairs your skin.

The reference website for dermatology
Share and develop your dermatological knowledge with professionals from all over the world.