NEOPTIDE Expert
Anti-hair loss and growth serum
NEOPTIDE EXPERT is indicated in the management of chronic alopecia in men and women (CTE, AGA, etc.).
Thanks to its exclusive patented active ingredients, it slows down hair loss, stimulates growth and increases the anchoring of the hair follicle in the scalp.
Clinical efficacy has been demonstrated in use alone and in combination with minoxidil.
This serum can also be prescribed to accompany hair transplants.
-
For whom?WomenMenTransplant
-
Price
-
TextureGel texture, fragrance-free.
Patient relations
-
Application tips
Hair loss:
- Once a day, 8 sprays (2 / parting) on dry or wet scalp.
- Massage the product into the skin with your fingertips.
- Do not rinse.
-
In combination with the following treatments
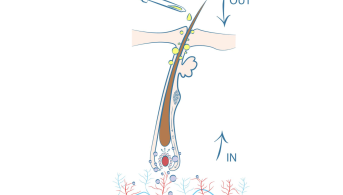
Minoxidil 2% & 5% topical solution.Hair transplantation:○ 15 days before and up to the transplant day, 8 sprays / day.○ 4 days after the transplant, 8 sprays to a piece of gauze and dab onto the transplanted area for 15 days.○ After complete healing, using the fingers, 8 sprays to the transplanted area. Massage gently.Use daily for at least 9 months.
Ingredients
Active ingredients:

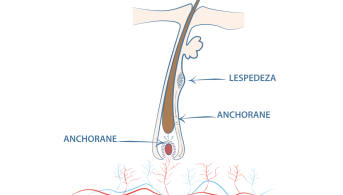
Anchorane®, a patented plant active ingredient, strengthens hair anchorage by stimulating the synthesis of KERATIN 75 (in vitro test: significant increase in the expression

Active ingredient of 100% natural origin, patented, dependent on dose, it significantly decreases the synthesis of the DKK1 protein (Dickkopf-related protein-1 = inhibitor of the Wnt pathway which favors pas

Mineral active ingredient, which significantly stimulates, in a dose-dependent manner, the synthesis of Versican by the cells of the dermal papilla (in vitro test): anchoring of the hair follic
Formula details:
Click on an ingredient type to highlight it in the list
Clinical results and publications
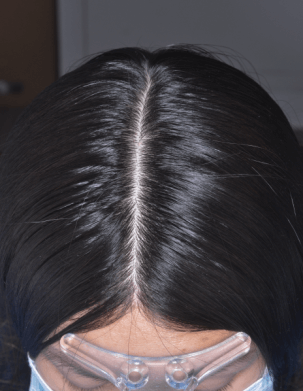
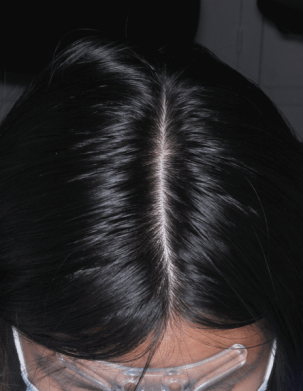
Efficacy results
From 4 weeks, versus control group (2).
From 4 weeks (3).
Increased hair anchorage at 2 months (4).
Hair in anagen phase (5).
Improvement in the feeling of hair anchoring in the scalp at 3 months (6).
Tolerance results
Tolerance only:
Very good skin tolerance(1).
Tolerance in combination with other treatments:
Tolerance validated with Minoxidil topical 2% & 5%.
Legal Notice
Clinical study of tolerance and effectiveness in 64 women with chronic telogen effluvium, under dermatological control. Randomized, controlled study versus untreated control group. Sabouraud Center.
(1) Skin tolerance rated by investigators.
(2) Significant decrease in the number of hairs removed in a pull test at the vertex area as early as S4 (p<0.05).
(3) Hair quality self-assessment questionnaire. % change vs. S0.
(4) 60' hair count: superior to control group at 4, 8 and 12 weeks (p<0.05).
(5) Ex vivo test performed on hair follicles with NEOPTIDE EXPERT vs untreated group.
Consumer test conducted on 137 adult subjects with chronic telogen effluvium. 1 application per day for 12 weeks
(6)% satisfaction.
Contact us
Would you like to meet a sales representative?
Our medical sales representatives come to your workplace to answer all your questions about our brands and products.
The product concerned may not be available in your country. Please contact your medical sales representative for further information.
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.