Summary of clinical results

SUNSIMED KA SPF 50+ formula remains on the skin's surface
Ex vivo study on human skin explants.
Population
3 adult donors of 9 skin explants.
SUNSIMED KA SPF 50+ application
1 single standardized application (2 mg/cm²).
Evaluation criteria
Quantification of the surface area occupied by the formula on skin explants 2h, 4h, 6h, 8h and 24h after application; comparison with an untreated control area. Image capture by C-cube camera.
Results
Stability of the surface area occupied by the product on the skin for 24 hours (non-significant difference versus T0).
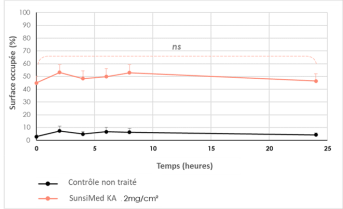
Change in surface area occupied by formula for 24h after a single application, versus untreated control area
ns : not significant (p> 0.05, versus T0)

Evolution of the surface occupied by the formula during 24h after a single application, versus untreated control area - C-Cube camera
Conclusion
The SUNSIMED KA SPF 50+ formula remains on the skin's surface for up to 24 hours.
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.