Summary of clinical results

Dexeryl emollient cream, an effective treatment for the symptoms of dry skin in ichthyosis
Objective
Demonstrate the efficacy of Dexeryl Emollient Cream in reducing the severity of ichthyosis.
Study
Phase III, international, multicenter, randomized, controlled, double-blind, parallel-group study: Dexeryl Emollient Cream versus vehicle (placebo).
Population
231 children included with a mean age of 8.3 years, suffering from a non-bullous form of ichthyosis: ichthyosis vulgaris (63.2%), X-linked recessive (14.7%), other (22.1%).
Dosage
2 applications daily.
Duration
12 weeks (4-week double-blind period with Dexeryl Emollient Cream or vehicle) followed by an open-label period with all patients treated with Dexeryl Emollient cream (for 8 weeks).
Primary endpoint
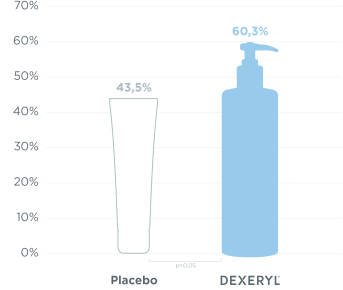
Improvement of ichthyosis by monitoring the evolution of skin xerosis via the SRRC* score: percentage of patients with a 50% reduction in SRRC score at D28.
Secondary endpoints
Pruritus: assessed using a visual analog scale (VAS). Global assessment (hydration capacity, improvement in perspiration): performed by each investigator and patient.
Tolerance
Twice-daily application of Dexeryl emollient cream to children's skin for 3 months produced no systemic or local adverse effects. Dexeryl emollient cream therefore proved safe and had a good tolerance profile.
Conclusion
Dexeryl emollient cream showed a significant reduction in xerosis1 in ichthyosis.
60% of patients significantly reduced symptoms by 50% in 28 days1
92% of patients rated hydration as good or very good1,2
40% of patients considered that sweating had improved slightly or very much.
80% of doctors in the clinical study found Dexeryl Emollient Cream effective in the management of ichthyosis in children1,2
Publication
1 - Blanchet-Bardon C et al. Association of glycerol and paraffin in the treatment of ichthyosis in children: an international, multicentric, randomized, controlled, double-blind study. JEADV 2012
2 - After 3 months of use.
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.