Summary of clinical results

Clinical study of the safety and efficacy of KELUAL DS soothing cream in subjects with seborrheic dermatitis.
Dermatologically controlled clinical study of the safety and efficacy of KELUAL DS Soothing Face Cream on teenagers and adults with mild to moderate seborrheic dermatitis of the face.
Population
60 subjects included
30% women/70% men (13 - 64 years) with mild to moderate seborrheic dermatitis of the face
- Erythema ≥ 1
- Pruritus ≥ 1
- Desquamation ≥ 1
Application of KELUAL DS Soothing Face Cream
1 single application per day in the attack phase, then twice a week in the maintenance phase
Evaluation criteria
- Clinical score by investigator: desquamation, erythema (scale from 0 to 4)
- Scoring by subject: average pruritus (analog scale from 0 to 10)
- Samples + qualitative metagenomic analysis by Haut sequencing of fungal and bacterial populations including Malassezia globosa, Malassezia restricta, Staphylococcus epidermidis, Staphylococcus aureus and Cutibacterium acnes
Results
Highly significant reduction in clinical signs after 7 days of treatment: desquamation, pruritus and erythema.
Intra-group analysis, **: p<0.001
Evolution of the clinical desquamation score
during 15 days of treatment and during a 6-week maintenance phase
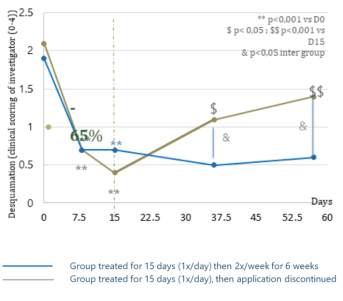
- Average pruritus: -62% after 7 days of treatment
- Erythema: -35% after 7 days' treatment
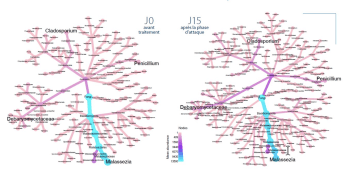
After 15 days of treatment, the majority Malassezia population is reduced in favor of new fungal families: Penicillium, Cladosporium and Debaryomyces.
Evolution of the fungal microbiome after 15 days of treatment
Good skin tolerance
Conclusion
After 15 days of treatment with KELUAL DS Soothing Cream, improvement in clinical symptoms correlates with rebalancing of the fungal microbiome. The increase in microbiome biodiversity thus approximates that of a healthy scalp.
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.