Summary of clinical results

Clinical study on the safety and efficacy of SENSINOL Treatment Shampoo in adults
Dermatologically and ophthalmologically controlled clinical study of the safety and efficacy of SENSINOL Treatment Shampoo in adults with chronic scalp pruritus.
Population
33 subjects included.
73% women/27% men (22 - 68 years) with chronic scalp pruritus (> 6 months)
- 3S score ≥ 4
- Pruritus score ≥ 2
Application of SENSINOL Treatment Shampoo
At least 3 applications a week for 21 days, followed by a 14-day residual phase with applications of the usual shampoo.
Evaluation criteria
- Scoring by subject:
◦ Self-assessment questionnaire of the impact of 5 sensations (itching, tingling, pulling, pain, burning) on quality of life (3S Score).
◦ Self-assessment of intensity of pruritus, pain and burning sensations (visual analog scale from 0 to 10) - Skin and eye tolerance
Results
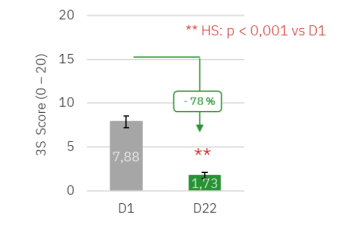
Highly significant reduction in the impact of scalp pruritus on quality of lifeafter 21 days of treatment : overall 3S score reduced by 78% at D22 (p < 0.001 vs. D1).
Evolution of the 3S score during the 21-day treatment phase
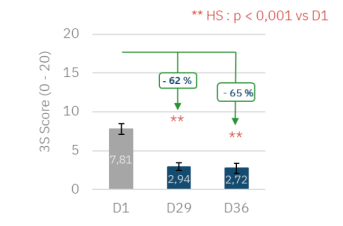
Continued improvement in the impact of scalp pruritus on quality of life 14 days after treatment discontinuation: global 3S score reduced by 62% at D29 and 65% at D36 (p < 0.001 vs. D1).
Evolution of the Global 3S score during the 14-day maintenance phase
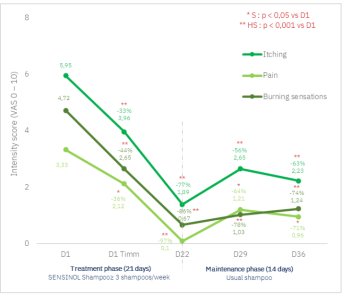
Significant reduction in the intensity of pruritus and signs of discomfort on the scalp (pain and burning sensations) from the 1st application of SENSINOL Treatment Shampoo and maintenance of the soothing effect 2 weeks after treatment discontinuation.
Evolution of the intensity score of discomfort sensations on scalp (VAS 0-10) during the during the 21-day treatment phase and the 14-day maintenance phase
Good skin tolerance and very good ocular tolerance.
Conclusion
SENSINOL Treatment Shampoo: soothing effect on pruritus and scalp discomfort from1st application. Significant improvement in quality of life associated with scalp pruritus after 3 weeks of treatment, with effect maintained 2 weeks after treatment discontinuation.
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.