Summary of clinical results
DUCRAY

Study of skin penetration of aluminum salts contained in the HIDROSIS CONTROL antiperspirant roll-on formula
In vitro study of skin penetration of aluminum salts contained in the HIDROSIS CONTROL antiperspirant roll-on formula
Population
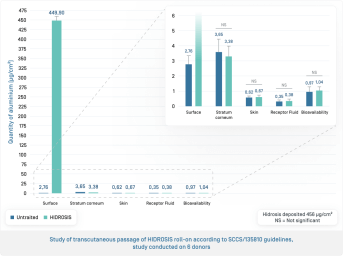
10 human skin samples from 6 different donors
Application of HIDROSIS CONTROL antiperspirant roll-on
- 1 application on skin samples
- Comparison to untreated samples
Evaluation criteria
- Quantification of the penetration of aluminum salts in each layer of the skin 24 hours after application
- Evaluation of the bioavailability of aluminum salts
Results
- The majority of the aluminum salts remain on the surface of the skin 24 hours after application of the product
- The quantities of aluminum salts found in the stratum corneum and the skin are very low 24 hours after application of the product
- The bioavailability of aluminum salts is 0.23% of the applied dose, with no significant difference compared to untreated samples
Conclusion
Very low skin absorption of aluminum salts
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.