Summary of clinical results
DUCRAY

Tolerance and efficacy of HIDROSIS CONTROL antiperspirant roll-on
Tolerance and efficacy of HIDROSIS CONTROL antiperspirant roll-on in adult subjects with underarm hyperhidrosis, under dermatological control
Population
34 adult subjects with underarm hyperhidrosis
1/3 of subjects with sensitive and/or dry skin
Application of HIDROSIS CONTROL antiperspirant roll-on
Application once a day for 9 days, compared to a market reference
Evaluation criteria
- Evaluation of antiperspirant efficacy: gravimetric measurement (weighed) at D0, D3, D8 and D10 of perspiration from cotton placed under the arms, before and after repeated exposure in a hot atmosphere (sauna)
- Evaluation of the persistence of the antiperspirant efficacy 48h after stopping application
- Skin tolerance
Results
- From 3 days, significant decrease in the quantity of sweat of –35% (p<0.01)
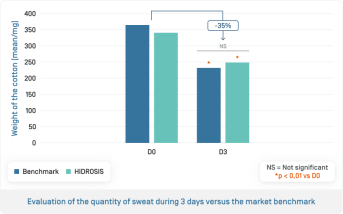
- Anti-perspirant efficacy equivalent to the market reference
- Antiperspirant effect persists for 48 hours
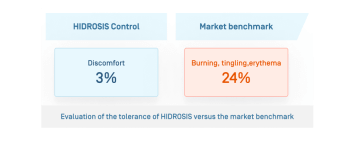
- Better skin tolerance than the market reference
Conclusion
- Antiperspirant efficacy from 3 days
- 48H efficacy
- Very good tolerance
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.