Publication Summary
Tolerance and efficacy of a new celastrol-containing balm as adjunct care in psoriasis
Objective
Evaluate the tolerance and efficacy of KERTYOL PSO Balm, containing Celastrol, an active ingredient with immunomodulatory properties in the Th17 pathway, and Polidocanol, an antipruritic active ingredient, in psoriatic patients
Methodology
2 open-label clinical trials:
43 psoriatic patients with mild-to-moderate plaques using KERTYOL PSO Balm alone (PASI Score= 2.5, Pruritus NRS Score=5.6)
51 treated psoriatic patients who used KERTYOL PSO Balm in combination with topical and systemic treatments
Application of KERTYOL PSO Balm to the entire body, once a day for 4 weeks
Evaluation Criteria
- Assessment of the tolerability of KERTYOL PSO Balm used alone or in combination with medicinal treatments (corticosteroids, vitamin D3, phototherapy, acitretin, biotherapy)
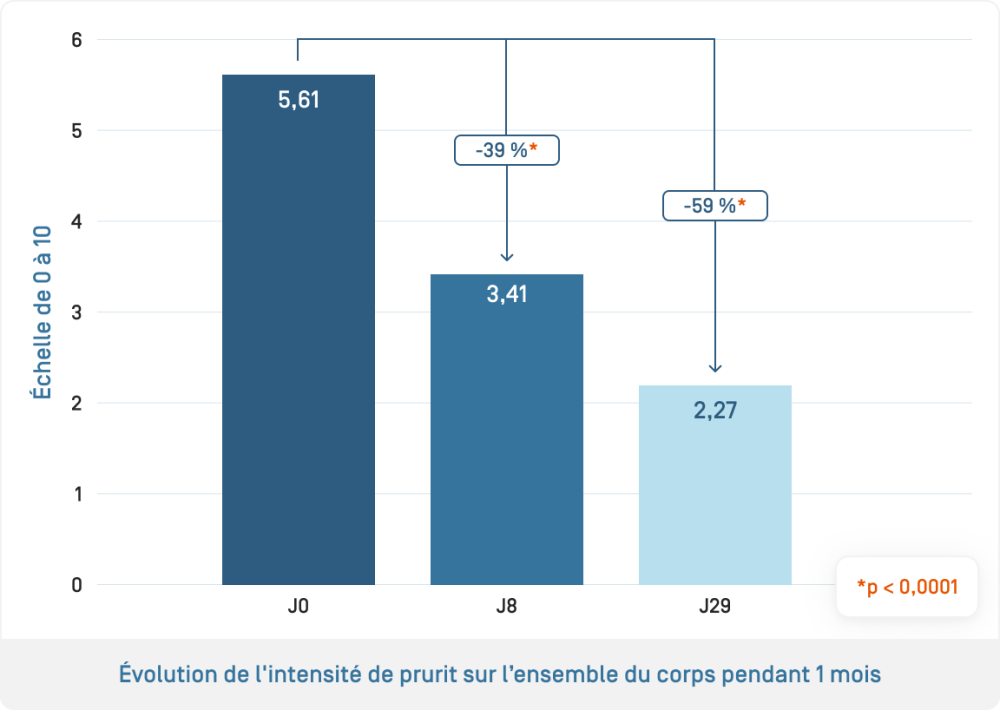
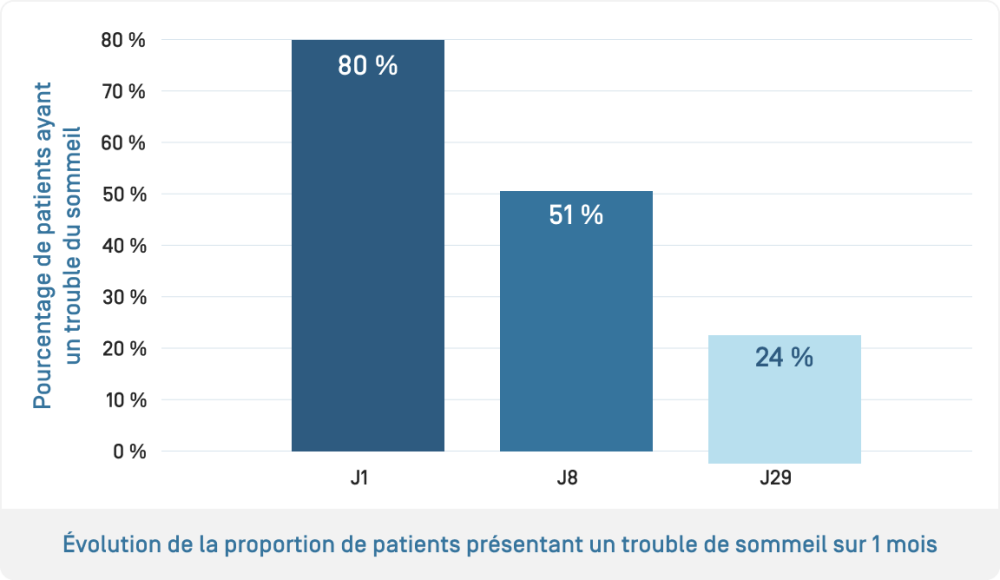
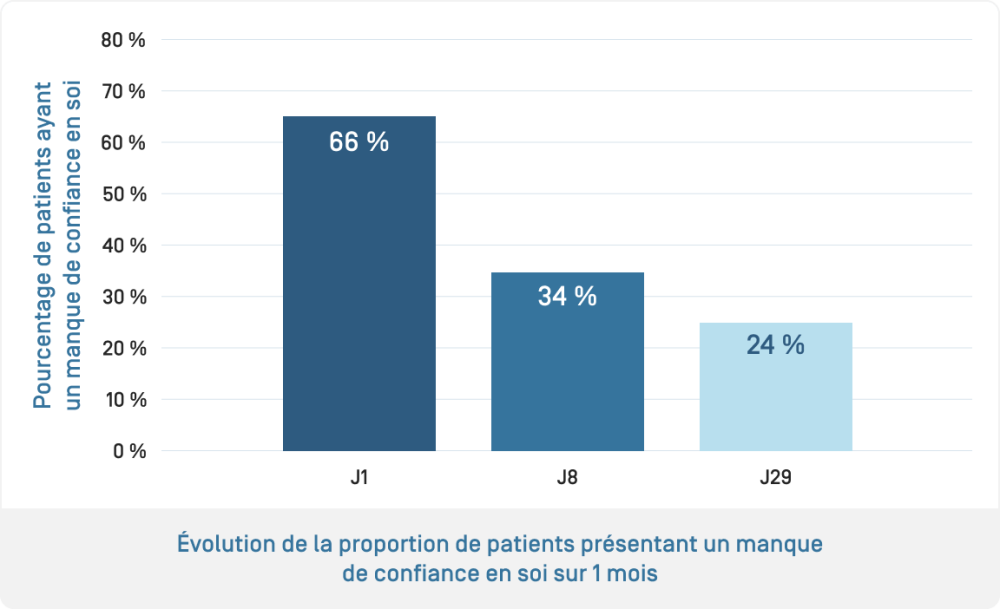
- Evaluation of the efficacy of KERTYOL PSO Balm used alone: measurement of pruritus intensity and its impact on quality of life using a patient questionnaire (PRO, Patient-reported "Burden of Itch Scale") to assess the impact of pruritus on daily life (activities, sleep, mood, etc.)
Conclusion
Excellent skin tolerance of KERTYOL PSO Balm alone or in combination with psoriasis treatments
The benefits of KERTYOL PSO Balm as a complementary treatment for the physical signs of psoriasis, pruritus and quality of life
Our other publications on this subject
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.