Summary of clinical results

Efficacy of a comprehensive supportive skin care program with hydrotherapy after treatment of non-metastatic breast cancer: a randomized controlled trial
The efficacy of post-treatment hydrotherapy as supportive care for the management of dAE with supportive hydrotherapy care
Population
70 women aged ≥18, in complete remission for non-metastatic invasive breast carcinoma, expressing hormone receptors but not overexpressing HER2, with or without invasion with or without lymph node involvement, and previously treated with surgery (including surgery or mastectomy with axillary or sentinel lymph node dissection), chemotherapy and radiotherapy), chemotherapy and radiotherapy
Study design
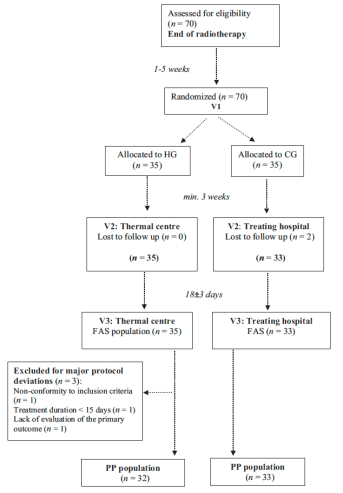
Patients randomized into 2 groups:
- A group receiving a 3-week hydrotherapy course of treatment at the Avène Hydrotherapy Center
- A group without hydrotherapy treatment
3 visits: V1 being for inclusion and randomization; V2 being the study start visit, and V3 being the end of the hydrotherapy treatment, 18 days later (+/- 3 days)
Evaluation criteria
- Changes in quality of life evaluated with the QLQ-BR23 questionnaire
- Clinical classification of dAE, using NCI-CTCAE: dermatitis radiation, dry skin, hand-foot syndrome, lymphedema, nail toxicity, skin pain, pruritus, skin hyperpigmentation, skin induration.
- Quality of life using the DLQI questionnaire
- Evaluation of cancer-related quality of life with the QLQ-C30 questionnaire
- General psychological well-being using the Psychological Global Well-Being Index (PGWBI)
Results
- Significant improvement in quality of life (DLQI) of 82% for spa patients
- Improvement in xerosis for 100% of hydrotherapy patients
- Good to very good tolerance
Conclusion
This study showed that Avène hydrotherapy treatment reduced the side effects of non-metastatic breast cancer treatment and improved patients' quality of life.
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.