Summary of clinical results

Efficacy and tolerability of KERACNYL serum in mild to moderate adult female acne
Population
33 female subjects aged 18-38 years old included in a 8 week open clinical study
Application of KERACNYL serum
2 applications a day (morning and evening)
Assessment criteria
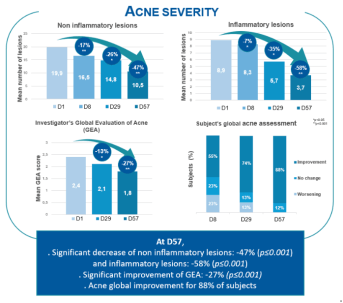
- Acne severity
- Acne lesions counting according Lucky method
- Global acne assessment (GEA)
- Global acne assessment by subjects
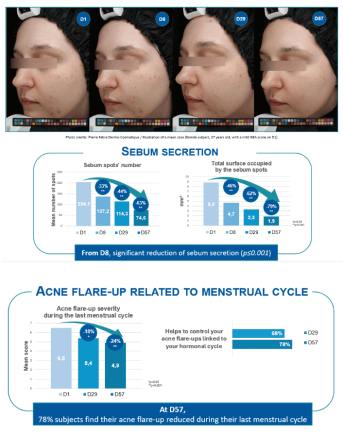
- “Acne flare-up” severity during menstrual cycle (subject self-assessment) – 10-point scale
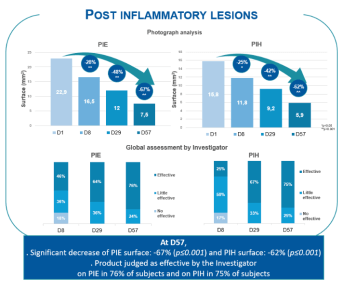
- Post-Inflammatory Erythema (PIE) and Hyperpigmentation (PIH)
- Surface according to photograph analysis (target lesions)
- Global assessment by Investigator
- Sebum secretion
- Sebaceous secretion evaluation by Sebutape® number + total surface occupied by the sebum spots
- Quality of life
- Cardiff Acne Disability Index (CADI) questionnaire
- Cosmetic acceptability questionnaire
- Tolerability
- Standardized photographs
Results
Quality of life: from D29, a significant improvement of the quality of life (CADI score) was observed (p≤0.001).
Cosmetic acceptability: cosmetic qualities and efficacy of the product were appreciated by the subjects.
Tolerability: cutaneous tolerance was assessed as good by the dermatologist.
Functional and/or physical signs were mainly of mild intensity and occurred at the beginning of the study.
-
Conclusion
Efficacy results of this new dermo-cosmetic serum highlight the potential benefit of this product as complementary care in adult females with mild to moderate acne and prone to acne-flare-ups related menstrual cycle. The good tolerance, the improvement of the quality of life and the cosmetic qualities and perceived efficacy of the serum could ensure a good observance in the management of acne.
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.