Summary of clinical results
DUCRAY

Tolerance and efficacy of MELASCREEN Anti-Spots Concentrate
Tolerance and efficacy of MELASCREEN Anti-Spots Concentrate in subjects with superficial facial hyperpigmentation (lentigo, melasma, post-inflammatory hyperpigmentation related to acne), under dermatological and ophthalmological control
Population
- 55 adult women (22 to 63 years old) with superficial facial hyperpigmentation (lentigo [n=14], melasma [n=32], post-inflammatory hyperpigmentation related to acne [n=9])
- Phototypes II to V; All skin types
Application of MELASCREEN Anti-spots concentrate
- Application twice a day to the entire face, neck and décolleté for 8 weeks
- Photoprotection recommended in case of sun exposure
Evaluation criteria
- Clinical scoring of overall efficacy on hyperpigmentation by the investigators (score from 0 = ineffective to 3 = very effective)
- Spectrometric evaluation of pigmentation (ITA angle measurement) of a pre-selected hyperpigmented area compared to an adjacent non-hyperpigmented control area
- Perceived efficacy and cosmetic acceptability evaluated by the subjects (score from 0 to 10; percentage of satisfaction)
- Skin and eye tolerance
Results
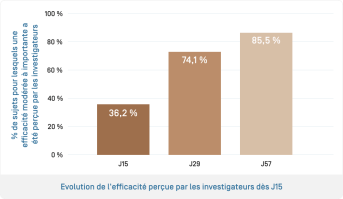
- Efficacy perceived by the investigators on the reduction of hyperpigmentation for 85.5% of subjects at D57
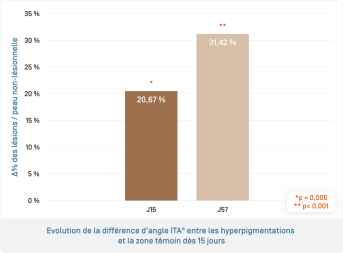
- Significant evolution (p=0.005) of the difference in angle ITA° between hyperpigmentation and the control area (= depigmenting and unifying effect) of hyperpigmentation from 2 weeks
- Efficacy perceived by the subjects: evenness of the complexion for 94% of the subjects at 1 month (average score of 8/10)
- Light, non-sticky texture for 100% of subjects (average score of 9.4 and 8.6 respectively) at 2 months
- Good skin tolerance and excellent eye tolerance
Conclusion
- Depigmenting efficacy and uniformity of the complexion
- Very good cosmetic acceptability
- Good tolerance
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.