Summary of clinical results

Ongoing efficacy in adolescents and adults with mild to moderate facial acne
Tolerance and efficacy of Comedomed + on acne-prone skin under dermatological and ophthalmological control
Population
41 subjects aged 13 to 26 (64% adolescents) with acne-prone skin and at least 5 inflammatory lesions, 10 non-inflammatory lesions and, for 50% of subjects, at least one post-inflammatory erythema and/or post-inflammatory hyperpigmentation.
Product application
Comedomed+ is applied 2x/day for 2 months all over the face.
Evaluation criteria
Subjects make 5 visits to the investigation center on D1, D6, D15, D29 and D57. During these visits, various criteria were analyzed:
- Dermatological and ophthalmological check-ups
- Lesion count
- Non-comedogenicity
- Lipid index assessments: sebum meter and sebum tape
Clinical evaluations :
- Pores
- PIE/PIH marks
- Redness and volume of 2 targeted papules
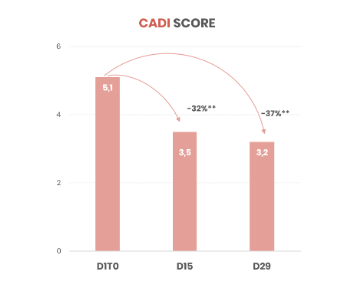
- CADI score
- Photos
- Cosmetic acceptability questionnaire
- Overall efficacy of product by investigators
- Overall efficacy on EIP/PIH by subjects and investigators
Results
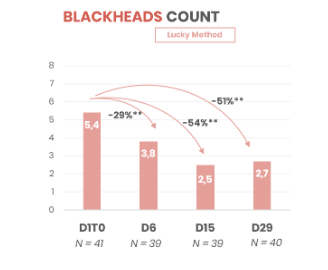
- Significant reduction in non-inflammatory lesions after 5 days of use
- Long-lasting effect up to 2 months
- 2 times fewer blackheads in 15 days of use
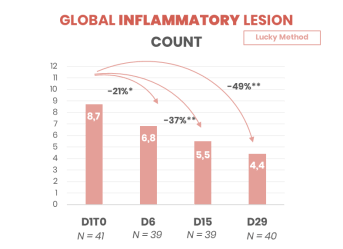
- Significant reduction in inflammatory lesions after 5 days of use
- Long-lasting effect up to 2 months
- 2x fewer inflammatory lesions in 1 month
Significant improvement in patients' quality of life after 15 days of use (based on CADI score)
Conclusion
Twice-daily application of CLEANANCE COMEDOMED+ Intensive Blemish Control Care demonstrated anti-acne efficacy on inflammatory and non-inflammatory lesions as early as 15 days, with clear improvement in 2 months. CLEANANCE COMEDOMED+ intensive anti-blemish care significantly improves patients' quality of life.
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.