Summary of clinical results

Open-label, single-center study Efficacy on target (infants, children, adults)
Objective
Evaluate the product's efficacy in patients suffering from atopic dermatitis.
Methodology
The study was carried out on 55 subjects (22 adults, 17 children, 16 infants) suffering from mild to moderate atopic dermatitis.
Patient characteristics
- SCORAD between 15 and 23.5 at inclusion
- Xerosis ≥ 1 (mild), with 47.3% of subjects with xerosis ≥ 2 (moderate)
- Pruritus ≥ 3 (on scale 0 to 10), with 89.1% of subjects with pruritus ≥ 4
- Usual emollient will be authorized if started at least 2 weeks before inclusion and unchanged and stable for the duration of the study.
The product was applied once or twice a day (depending on cleansing habits) to the face, body and scalp (for infants).
Study conducted under dermatological and pediatric control.
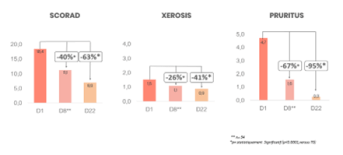
SCORAD evaluation at D1, D8 and D22
Results
Conclusion
XeraCalm AD Lipid-replenishing oil significantly reduces SCORAD and Xerosis and Pruritus sub-parameters after 8 days and 22 days of use. There was also a significant reduction in all of the following sub-parameters: erythema, papulation/edema, excoriation and lichenification.
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.