Summary of clinical results

Tolerance and efficacy of CLEANANCE COMEDOMED anti-blemishes concentrate as monotherapy - Comparative study
Tolerance and efficacy of CLEANANCE COMEDOMED anti-blemish concentrate as monotherapy in adults with mild-to-moderate, predominantly retention acne of the face, under dermatological control and compared with an untreated control group.
Population
36 subjects aged 18 to 35 years with mild to moderate facial acne predominantly retentional (≥ 10 retentional lesions ; ≤ 10 inflammatory lesions)
2 parallel groups: treated group (N= 18) versus untreated control group (N= 18)
Application of CLEANANCE COMEDOMED anti-blemishes concentrate
- Treated group: 2 applications per day on the face for 8 weeks
Evaluation criteria
- Lion count on the whole face (Lucky's method)
- Acne severity (Investigator global assessment - IGA)
Results
- Highly significant decrease in number of acne lesions versus untreated control group as early as D28 (p<0.001)
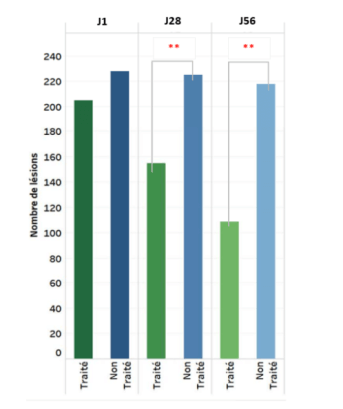
Change in number of total acne lesions over 8 weeks
** p= statistically Highly Significant (p<0.001, versus untreated group)
- Significant improvement in acne severity (IGA score) versus untreated control group as early as D28 (p<0.05)
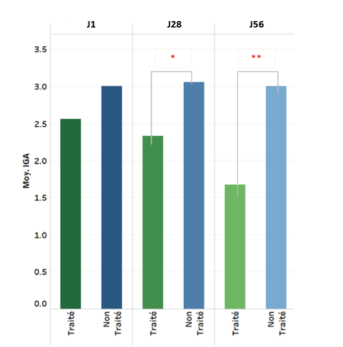
Change in IGA score (acne severity) over 8 weeks
* p= Statistically Significant (p<0,05, versus untreated control group)
** p= statistically Highly Significant (p<0.001, versus untreated group)
Conclusion
CLEANANCE COMEDOMED anti-blemishes concentrate applied twice daily demonstrated significant anti-blemish efficacy as early as 1 month compared to an untreated control group
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.