Summary of clinical results

Assessment of the skin and ocular acceptance of a topic use product through instrumental analysis, clinical efficacy and self-perceived efficacy by the subjects under normal conditions of use
Methodology :
Objective: To evaluate the acceptability of the product on the face, confirming the absence of comedogenic/ acnegenic potential, AEs and cutaneous and ocular discomfort sensations
Population: 33 adult subjects, mean age: 29 years old, phototype I to V
Acne prone subjects, presenting showing facial acne spots, lack of homogeneity/ uniformity in skin tone, enlarged pores, oily skin on the face
Design (application):
Once or twice daily application on the face, for 28 days
Evaluations:
Tolerance: dermatological and ophthalmological assessments
Efficacy evaluation through self-Assessment questionnaires
Evaluation of clinical efficacy by a dermatologist (assessment of acne severity (IGA), acne spots - PIH, pore opening, texture and homogeneity of skin tone)
Skin oil assessments: Sebumeter®
Evaluation of the product efficacy in inflammatory and non-inflammatory acne vulgaris: by counting lesions
Results:
After the 1st use, statistically significant decrease of
Sebum rate: -91%, with an improve in 100% of subjects
After 4 weeks of use (D29), statistically significant decrease of Sebum rate: -32%, with an improve in 91% of subjects
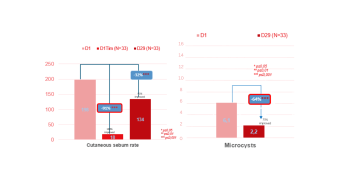
After 4 weeks of use (D29), statistically significant decrease of Microcysts*: -64%, for 73% of subjects
Conclusion:
- 91 % sebum form the first application
- 64% of whiteheads after 4 weeks of use
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.