Summary of clinical results
DEXERYL

In-use Tolerance and efficacy study under dermatological control of Dexeclear hydrating soothing cleanser on subjects treated by a topical or oral anti-acne medical treatment
Objective
- Assessment of the cutaneous tolerance of the Dexeclear hydrating soothing cleanser, after 28 days of use
- Assessment of the evolution of physical and functional signs after 7 and 28 days of use
- Evaluation of the Subjective efficacy and cosmetic acceptability of the product, after 7 and 28 days of use
Population
- 43 subjects: teenagers and adults (mean age : 18,3 years), presenting acne, skin dryness and erythema
- Treated with a topical or oral anti-acne medical treatment
- phototype II to IV
Application of DEXECLEAR Soothing Moisturizing Cleanser
Cleansing Cream, 2 times per day, for 28 days
On face, upper back and thorax, after wetting the skin and before drying it
Evaluation criteria
- Dermatological tolerance evaluation D1, D8 and D29 (5-point scale)
- Evolution of physical and functional signs at D8 and D29
- Cosmetic acceptability and perceived efficacy = Patient questionnaires at D8 and D29
Results
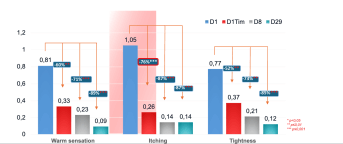
- Excellent skin tolerance
- After the first application (D1Tim), statistically significant decrease of itching perceived by the subject : -76%
- 88% of subjects find their skin soothed immediately
- 91% of subjects find their skin more comfortable and soothed after 8 days
Conclusion
- Excellent skin tolerance
- -76% itching sensation due to dry skin on face from the 1st application
- 88% of subjects find their skin soothed immediately
- 91% of subjects find their skin more comfortable and soothed after 8 days.
Results
After the first application (D1Tim), a very significant reduction in itching: - 76%.
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.