Summary of clinical results
DUCRAY

Tolerance and efficacy of KERACNYL PP+ anti-blemish cream
Tolerance and efficacy of KERACNYL PP+ anti-blemish cream in monotherapy in adolescent and adult subjects with mild to moderate acne, under dermatological and ophthalmological control
Población
41 subjects aged 12 to 35 with mild to moderate facial inflammatory acne, and at least one post-inflammatory erythema (PIE) and/or post-inflammatory hyperpigmentation (PIH)
Application of KERACNYL PP+ anti-blemish cream
Application twice a day (morning/evening) to the entire face for 8 weeks
Evaluation criteria
- Clinical scoring of efficacy on acne lesions: Lucky Method acne lesion count
- Evaluation of efficacy on residual marks due to inflammation and hyperpigmentation (PIE and PIH)
- Skin and eye tolerance
Results
- From 7 days, significant decrease in the number of total inflammatory lesions, which continues to reach –41% after 8 weeks
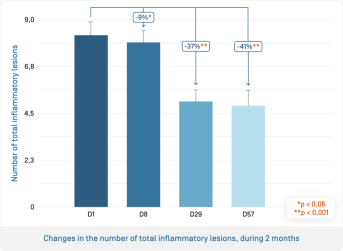
- From 7 days, significant improvement of post-inflammatory erythema
- At 1 month, significant decrease in the surface area of post-inflammatory hyperpigmentation lesions of -52%
- Very good skin and eye tolerance
Conclusion
- Efficacy on inflammatory lesions from 7 days
- Anti-mark action
- Very good tolerance
More summaries of clinical results
Want to read on?
This access is reserved for professionals, registered on Pierre Fabre For Med.
To access the full content, please register or log in if you already have an account.